
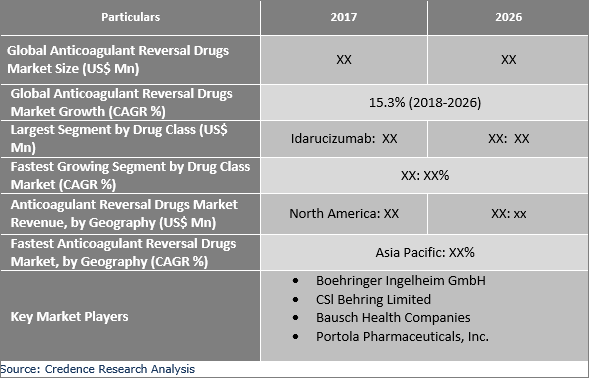
and continues to grow at a significant rate. "The number of Europeans taking a Factor Xa inhibitor is nearly double that of the U.S. Portola is executing a phased launch of Ondexxya in Europe, with an initial focus on Germany, Austria, the United Kingdom, the Netherlands, Sweden, Denmark and Finland, where Factor Xa inhibitor use, and related bleeds, are among the highest in Europe. These sales mark the initiation of commercial access in Europe to Ondexxya – the first and only reversal agent approved for adult patients treated with the Factor Xa inhibitors rivaroxaban or apixaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. ® (Nasdaq: PTLA) today announced the Company's first sales of Ondexxya ® (andexanet alfa) in Europe.
6, 2019 /PRNewswire/ - Portola Pharmaceuticals, Inc.


 0 kommentar(er)
0 kommentar(er)
